Clean chemistry, polymer science and a patented process, create the EMPEL™ advantage
The EMPEL™ chemistry foundation
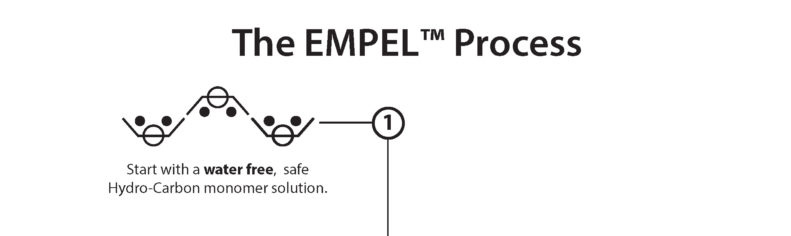
Clean Chemistry, the foundation to a better long-term solution: use Hydrogen and Carbon – the building blocks of everything found in nature – and eliminate the use of water.
In traditional textile manufacturing, chemicals known as polymers are dissolved in water and then washed into the fabrics with a variety of complex machines. The problem with the old process, chemicals that wash on with water wash off with water. These water-based chemistries lack durability, uniformity and often involve many volatile chemicals dangerous to our health and our planet.
GTT starts with a water-free nature-based Hydrocarbon monomer solution. The best analogy is a can of white paint. Like adding color to a can of paint, additional safe chemistries can be added to the GTT base chemistry and then molecularly bonded to each fiber with a process called polymerization.
Conclusion; Fabrics treated with the GTT Hydrocarbon chemistry outperform all existing industry standards and eliminate the toxic waste stream discharged when polluted factory water gets dumped into the environment.


Polymers, the building blocks of GTT
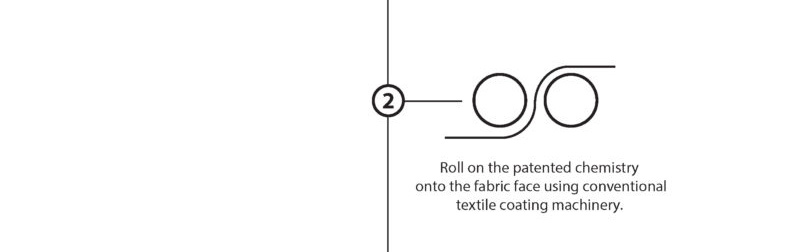
The EMPEL™ process takes simple monomers and bonds them together as performance enhancing polymer chains to encapsulate each fiber of the fabric.
The word Polymer has two Greek roots, Poly meaning many and meros meaning parts. Polymers are large molecules made of small, repeating molecular building blocks called monomers. The process by which monomers are linked together to form a long molecule of a relatively high molecular mass is known as polymerization.
Molecular Bonding, why our chemistry lasts longer, performs better and creates new product opportunities
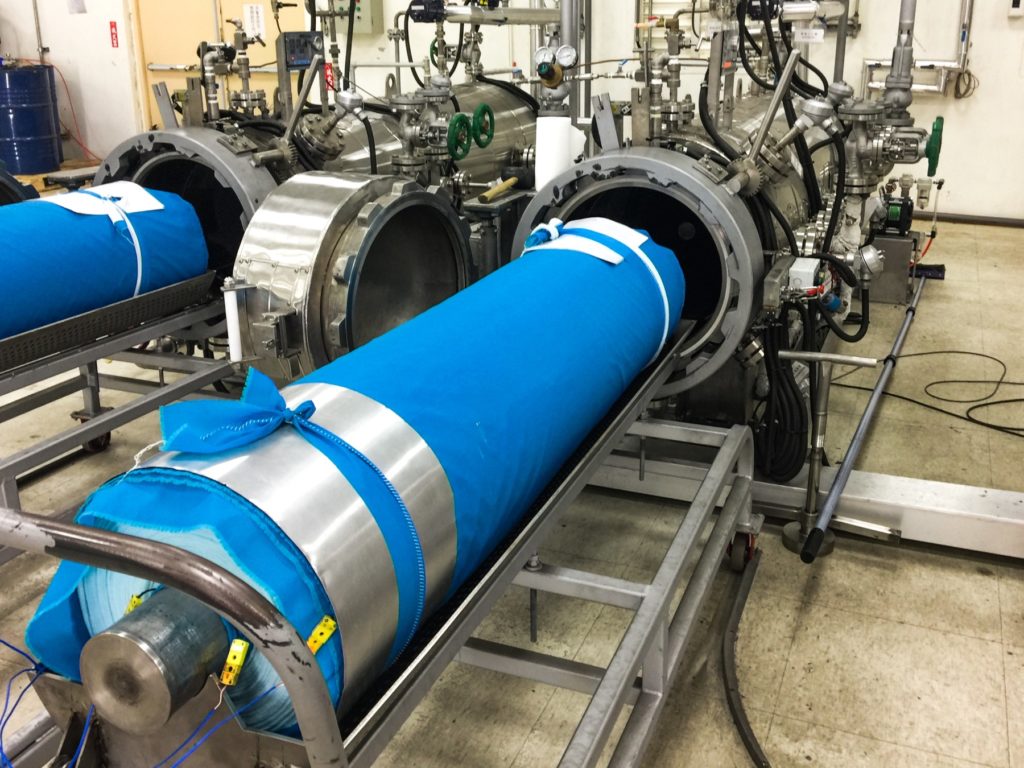
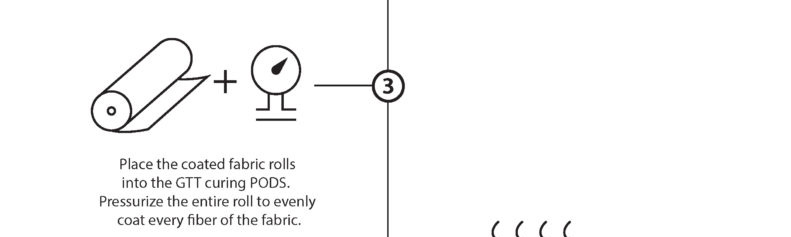
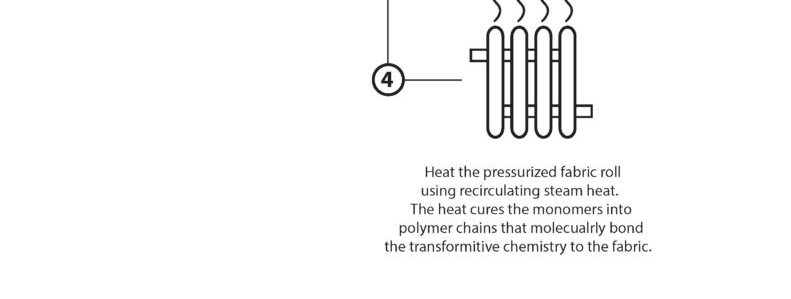
“Dry Curing” is the water-free, solvent-free process of taking our base monomer chemistry and turning them into polymers at the sight of the fibers. The EMPEL™ process uses thermal curing to create a molecular covalent bond between monomers and the fibers in the fabrics treated with our patented “Clean Chemistry”. Thermal curing is accomplished by passing EMPEL™ treated fabric through a heated zone. The heat that the fabric is exposed to causes molecular bonding and polymerization to occur.
Scanning Electron Microscope Pictures
| EMPEL Dry Finish | Traditional Wet Finish |
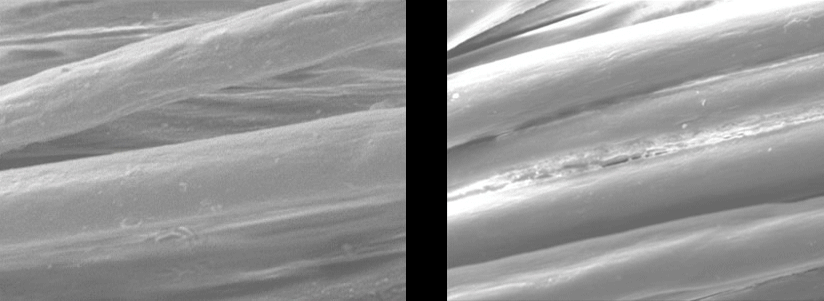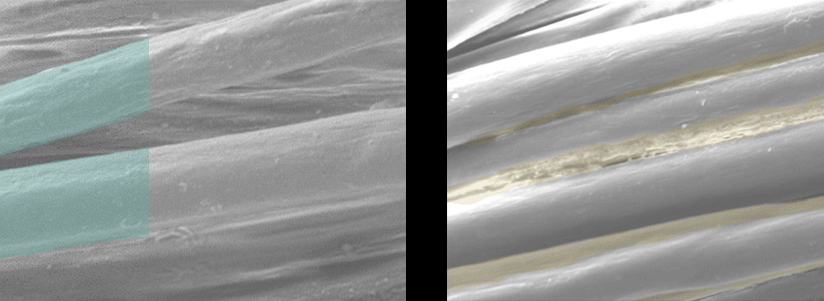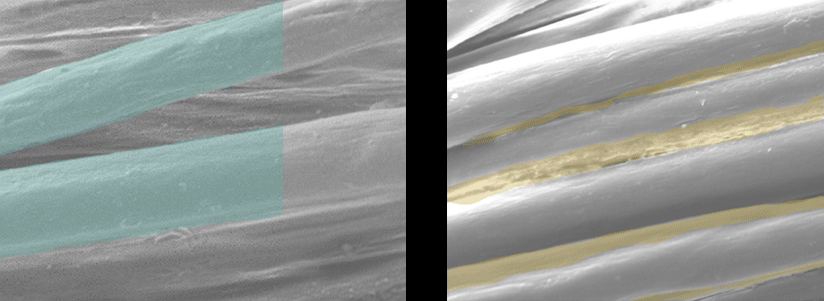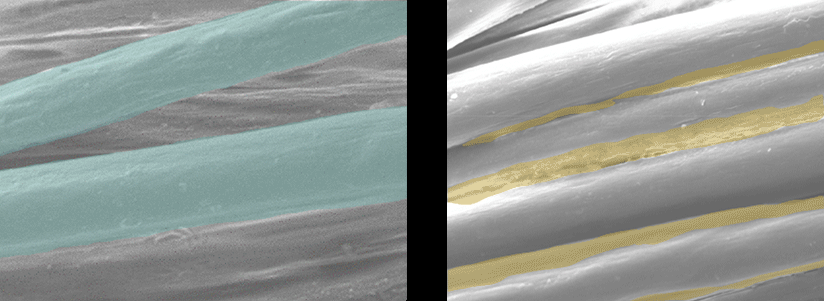
Each fiber is individually coated

Test Results
Rigorous testing shows that EMPEL™ outperforms all other water repellent finishes.
There are two compelling reasons EMPEL™ Molecular Water Protection should replace all other water repellent finishes on the market; it delivers 10X better Performance and Durability. We test EMPEL™ against C8, C6 and C0 padded wet finishes for water repellency over time, laundry durability and abrasion resistance. The results are always the same, EMPEL™ wins, without close competition.


Bundesmann testing shows that EMPEL™ delivers amazing results
Bundesmann test results also measure three levels of performance:
- Water beading on the surface is measured on a scale from 1-5. 5 means almost no drops are left on the surface after a 10-minute rain shower. 1 means the fabric has failed completely.
- Any water passing through the fabric swatch is collected in the cup and measured in milliliters.
- The test fabric swatch is weighed before and after the test to determine how much water is absorbed by the fabric structure.
EMPEL™ versus C8, C6, not a contest
Here is the PROOF that EMPEL™ delivers better results than all other water repellent finishes. First, for performance, we directly compare fabrics treated with the leading toxic Fluorine chemistry C8 and C6. Several leading brands still use the C6 finish although the chemistry is hazardous and not as effective as the GTT EMPEL™ process.




